Put your protocol to work
The foundation you need for compliant, collaborative trials
More than a document. Your protocol is a dynamic engine for action.
Protocols shouldn't sit in folders, they should drive action. HumanTrue transforms static PDFs into structured, interactive content that teams can search, interpret, and use in real time. No more version confusion, endless email threads, or buried details, just clarity, momentum, and alignment from start-up to submission.
When your protocol becomes a living tool, progress isn't just possible, it's built in.
Secure, Trustworthy, Enterprise AI
The foundation you need for compliant, collaborative research.
HumanTrue delivers a secure and transparent platform tailored for regulated, collaborative research, with enterprise-grade protections, robust validation, and audit-ready design built into every interaction. It transforms existing protocols into interoperable, CDISC-aligned formats automatically, eliminating manual conversions while integrating naturally into customers' systems for example, exporting a structured Schedule of Activities (SoA) to IRT or CTMS platforms, or generating consent documents compatible with eConsent systems. Leveraging AI and structured data, HumanTrue ensures integration and quality assurance through real-time verification, human oversight, and specialized models, effectively minimizing hallucinations and ensuring reliable, compliant clinical trial insights.
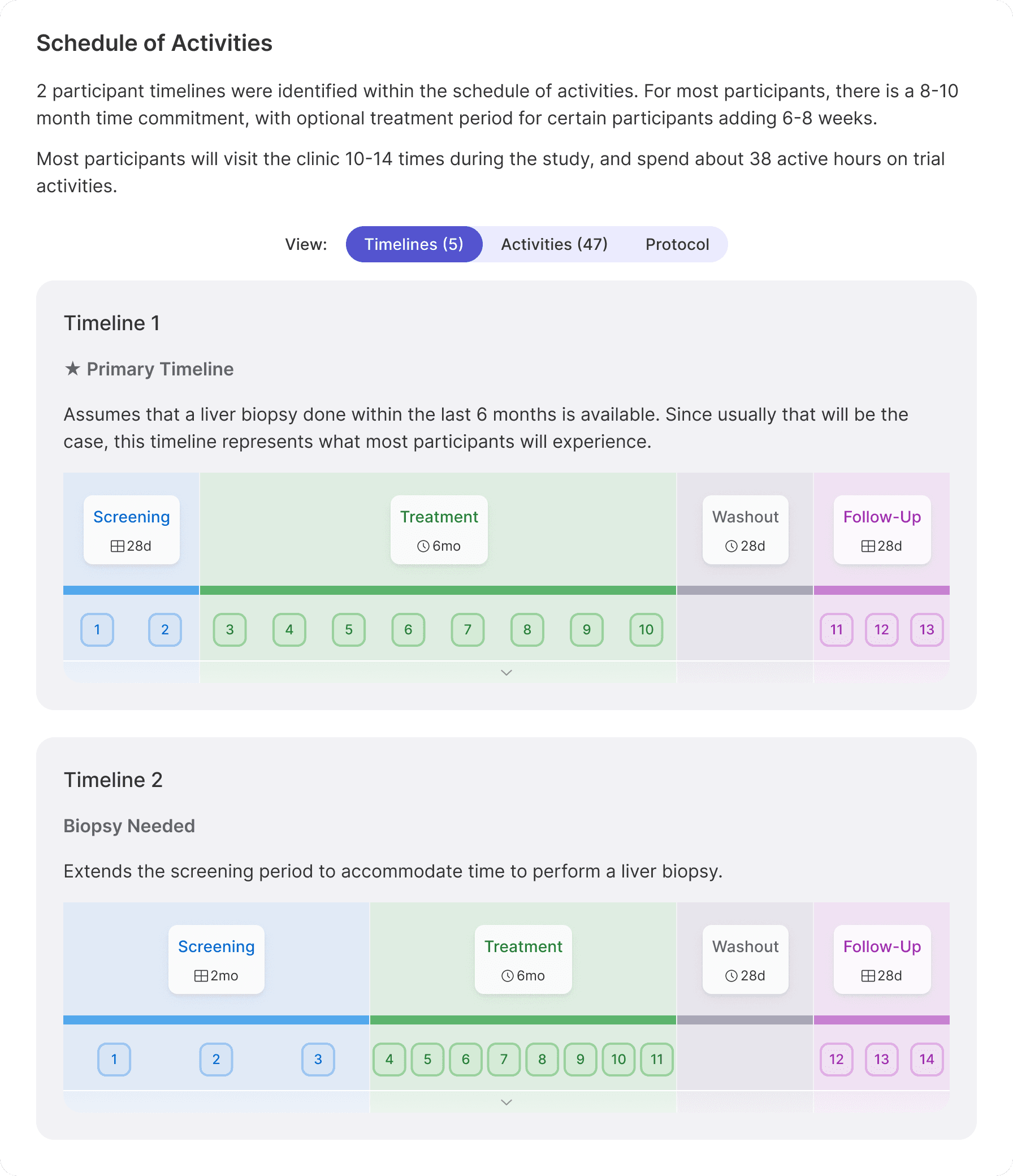
Fast start times
Despite protocol complexity, move faster... Navigating trial complexity with clarity, confidence, and collaboration.
Complex trials don’t have to mean chaotic operations. HumanTrue transforms protocols into structured, searchable content, making critical details easy to find, share, and act on. With standardized sections and tools built for high information density without the clutter, teams stay aligned and launch faster; no extra meetings, no endless email threads. Building on this foundation, HumanTrue also offers intelligent document generation aligned with regulatory guidelines, enabling rapid creation of essential materials like informed consent forms, case report forms, and recruitment screeners in minutes instead of weeks. This accelerates refinement, reduces IRB resubmissions due to overlooked details, and frees teams to focus on what matters most. Need translation? That’s built in too. Documents can be automatically translated within the system, complete with human certification aligned with ISO 17100 standards to ensure accuracy.

Enable your partners, reduce costs
Innovation lives in the details. Support shared understanding among CROs, sites, and participants.
When every detail counts, clarity becomes the foundation of a successful trial ecosystem. HumanTrue empowers every partner in the clinical trial pipeline, from sponsors and CROs to sites and participants, by translating complex protocols into accessible, role-specific insights. With contextual translation and multi-perspective views, each stakeholder sees the right information in the right way, fostering alignment, reducing friction, and accelerating execution. Because true innovation happens when your entire ecosystem speaks the same language.
ROI for Sponsors
Deliverable:
Informed Consent Drafting
Traditional Process:
2-3 days
With HumanTrue:
10-30 minutes
Impact:
90%+ time savings
Deliverable:
Site Training Guides
Traditional Process:
3-5 days
With HumanTrue:
< 1 hour
Impact:
95% time savings
Deliverable:
Patient Screener
Traditional Process:
2 days
With HumanTrue:
< 30 minutes
Impact:
85% time savings
Deliverable:
ISO 17100 Content Translation
Traditional Process:
3-4 weeks
With HumanTrue:
5-7 business days
Impact:
~75% time savings