Transform complex protocols into clear, actionable tools
Streamline operations, reduce costs, and get trials up and running faster using the HumanTrue study assistant.
The HumanTrue AI application understands clinical trial documents like you do, and will help your team understand protocols, share information, and even create new content like consent forms, recruiting screeners, and study guides. Oh, and it speaks any language.
Instant Protocol Clarity with your AI Study Assistant
Quickly grasp trial details, from basics to intricate eligibility criteria, with unlimited, natural language questions.
Your study expert with comprehensive insights
Understand study design, indications, tests and procedures in depth
Eliminate manual searches
Answers are instantly at your fingertips for all elements of trial (e.g. criteria, SOA, etc)
Centralized team collaboration
Keep everyone aligned with a source of truth.
Chat with your protocol
Ask unlimited questions, get great answers that are tailored to what you want to know
Built-in quality assurance
Real-time data checks, expert oversight, and specialized models work together with feedback loops and trusted sources to minimize hallucinations and ensure accurate, compliant, and reliable clinical trial insights
Make it your own
Every trial is unique, so we customize deliverables to align with your objectives, voice, and workflows, ensuring your protocol becomes an active tool for ongoing progress.
Generate Trial-Ready Documents, Faster and Flawlessly
Effortlessly create and tailor essential study documents, from patient materials to training guides, ensuring compliance and accelerating delivery.
Rapid document generation
Produce compliant study documents based on unique trial characteristics and existing templates.
Tailored content
Automatically adjust reading levels and generate recruiting like screeners, patient facing such as ICFs, and training materials.
Accelerated ISO 17100 compliant translations
Get certified content translated to any language 70% faster and more cost effectively than traditional methods.
Fast-tracked Insights
Quickly launch feasibility or coverage analyses.
Quick answers without the PDF overload
Stop wasting time manually searching through PDF files. HumanTrue understands protocols and also knows clinical research. We understand what it is you're looking for and generate insights within minutes of uploading a protocol.
Prepare for a study startup meeting by reviewing key details like phase, indication, number of participants, eligibility criteria, and the study goals.
Start a feasibility analysis by reviewing the schedule of activities.
Create a list of labs specified in the protocol and see if any parameters deviate from the standard.
Start your recruiting efforts by understanding the population characteristics and the specific indications studied.
Analyze patient burden by quickly looking at the number of clinic visits, procedures, and read the analysis of risks.
Use the protocol as a learning launchpad
Protocols are detailed specifications of clinical research, but might not contain all answers to your questions. Not only does HumanTrue understand a specific protocol, it understands medical research, classes of drugs, industry-standard tests, and more - everything that makes up the full context of a study.
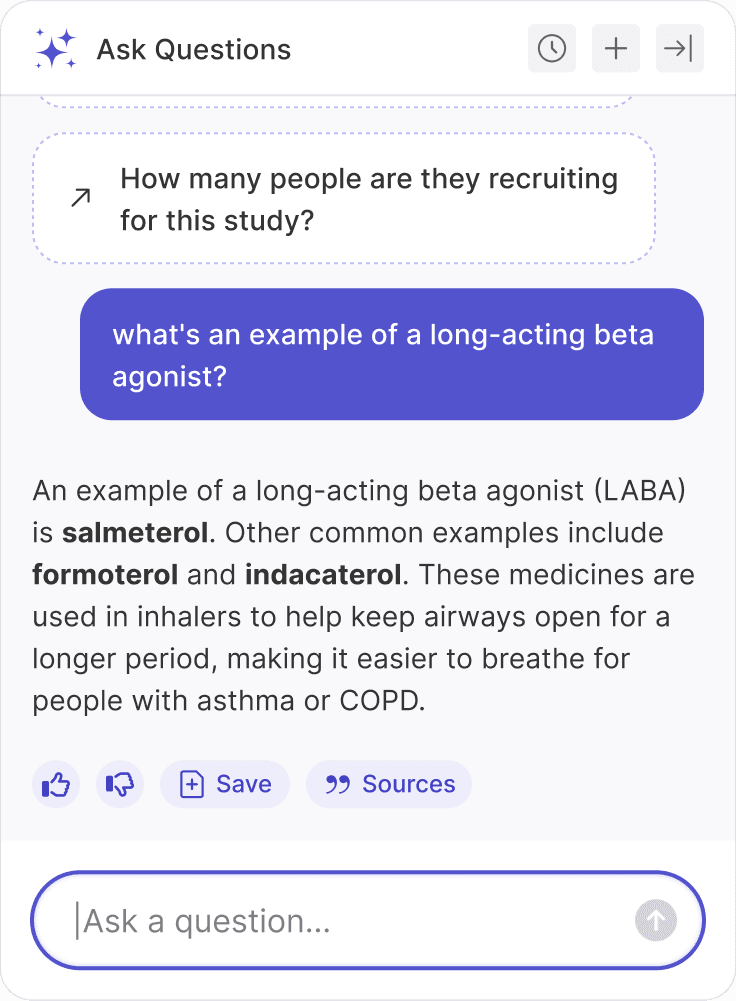
You ask the same questions, so automate them
Your organization or your team members likely review protocols for the same thing, multiple times a day. Recruiters look at population details. Phlebotomists look for labs and specimen collection procedures. Medical writers look to understand benefits and risks.
Use HumanTrue to not only automate answers to these questions, but to provide a consistent way to quickly see these details across a library of studies.
Share with your team
Sharing a PDF file over email isn't working anymore. Use HumanTrue to create a centralized, searchable, library of protocols with easy access to all members of your team.
Keep everyone on the same page by creating a simple, consistent way to view and understand protocol details. Share new insights or conversations with your team to create a running FAQ.
Digital Protocol
HumanTrue converts protocol documents to easily navigable digital formats, making it quick to find, identify, and utilize rich information essential to the study process.
Use the protocol to its full potential with a user interface that breaks it down into the sections that you want to see like exploring the schedule of activities for a budget analysis, evaluating the participation criteria and patient population for recruiting logistics or evaluating the risks and burdens for patients for a site feasibility analysis.
HumanTrue also makes the entire document fully interactive with integrated features such as linking to external databases for extra information/context, your 24/7 clinical trial chat expert and references to where to find information in the source protocol.
Mobile-friendly application, from any device
Work occurs everywhere, that's why HumanTrue works on mobile devices. Securely login and access your library of protocols, ask questions, or view generated content from your mobile device.

Don't write, refine
The HumanTrue platform excels at creating new content based on protocols. We believe that humans play an important (and ultimately, final) role in the creation and approval of clinical research documentation, but you can achieve your goals much faster with help from our platform.
HumanTrue can create trial-specific content like consent forms, recruiting materials, training materials, and even reports or analysis. In minutes, generate content that is verifiably accurate, meets regulatory guidelines, and is translated. Spend time refining, editing, and working with your team rather than weeks on a first draft.
Consent Forms
Generate regulatory-compliant and trial-aligned informed consent forms in minutes, not days.
Upload consent form templates - from the sponsors, sites or CROs - to generate location-specific forms.
Automatically validate generated content against regulatory requirements for compliance and accuracy.
Export consent content in ready-to-submit formats, or formats that match your internal workflow.
Translate content swiftly and precisely to support global trial execution.
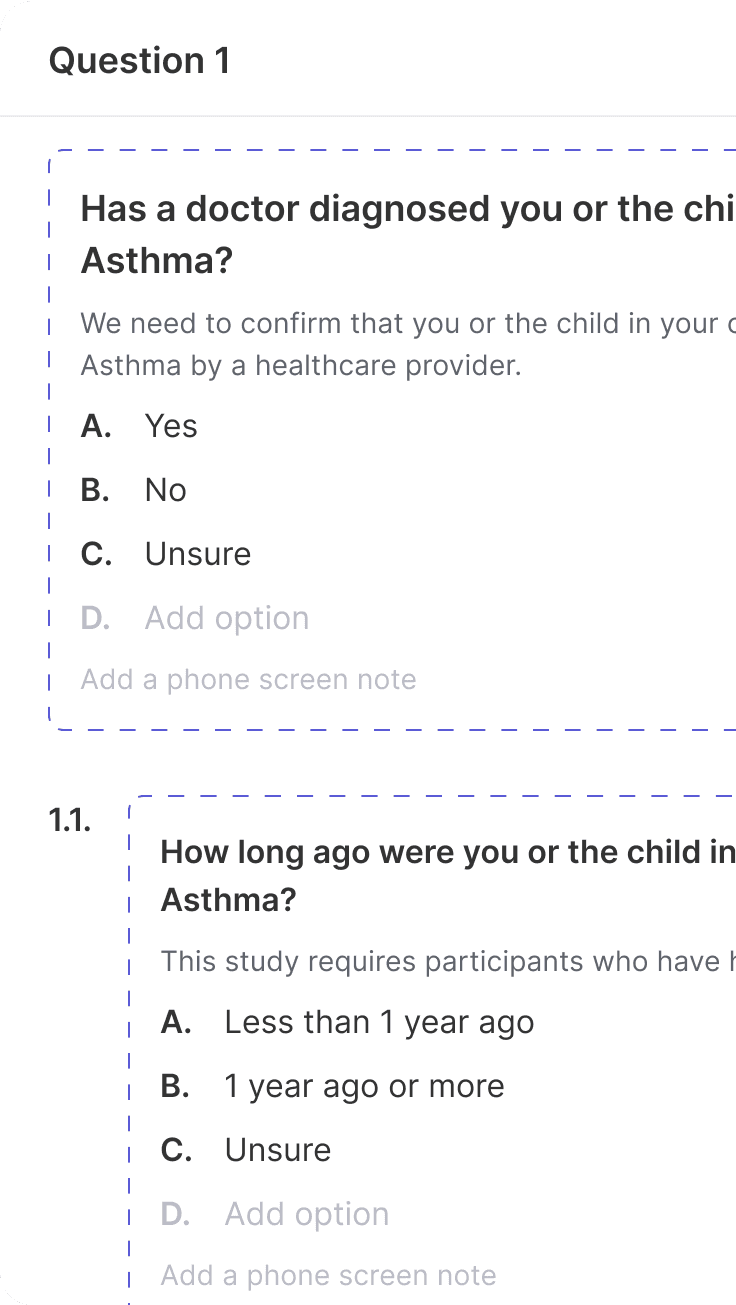
Recruiting Tools
Effortlessly compile comprehensive recruiting materials without worrying about missing key details from the protocol.
Produces screening materials with all of the unique aspects of the trial (e.g., criteria, population, etc.) in minutes.
Refine using built-in, simple editing tools.
Export ready for IRB submission or team review.
Translate content into needed languages for the trial.
Your Language. Your Reading Level. No Guesswork.
HumanTrue is designed for how you speak and read. It's also designed for how patients speak and read. The heart of the platform is its ability to adapt content to the specific language and reading level of each user, ensuring clear communication and understanding.
Achieve certified, multilingual content delivery in record time; at 70% less time and cost than conventional translation services. Upload content in any language. Communicate across languages and dialects with built-in multilingual chat. Adapt language to match specific reading levels, age groups, and comprehension needs. Whether it's language, dialect, or literacy our foundation is built to make your content accessible to everyone.

Platform and Services
Custom Output / Content for you
Because no two trials are the same, we partner with you to tailor outputs, whether regulatory documents, training guides, or patient materials, based on your goals, your voice, and your workflow. When your protocol becomes a living tool, progress isn't just possible, it's built in.
Engineered with Standards in Mind
HumanTrue understands the content of your protocols, without manual data entry or needing to fill in various forms. We do this by converting a protocol document to a semantic (AI) model, defined in part using industry standard data definitions.
Standards such as CDISC's USDM specification enable protocol data to be portable. We see standards such as this as a way to integrate with various systems and other vendors in the market.
Beyond standards, our use of AI and structured data under the hood means we can integrate with myriad customers systems. For example, our extracted Schedule of Activities (SoA) could be exported in a format compatible with IRT or CTMS systems. Generated consent documents could be exported to eConsent systems.